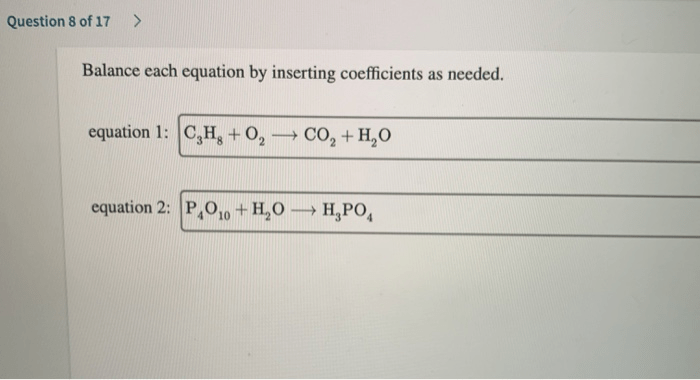
Pogil acids and bases answer key provides a comprehensive guide to the fascinating world of acids and bases, delving into their definitions, properties, reactions, and applications. This detailed resource empowers students and enthusiasts alike with a profound understanding of these fundamental chemical concepts.
Acids and bases play a pivotal role in numerous chemical processes, shaping our everyday lives and the natural world. Their significance extends from industrial applications to biological systems, making a thorough grasp of their behavior essential for scientific exploration and practical applications.
Acids and Bases Definitions
Acids and bases are two important classes of chemical compounds that play a crucial role in various chemical processes. They have distinct properties and undergo characteristic reactions.
The Arrhenius theory defines acids as substances that produce hydrogen ions (H+) when dissolved in water, while bases are substances that produce hydroxide ions (OH-) when dissolved in water. This theory, however, has limitations and cannot explain the behavior of acids and bases in non-aqueous solutions.
The Bronsted-Lowry theory extends the Arrhenius theory by defining acids as proton (H+) donors and bases as proton acceptors. This theory provides a more general definition of acids and bases and can be applied to both aqueous and non-aqueous solutions.
The Lewis theory further broadens the concept of acids and bases by defining acids as electron-pair acceptors and bases as electron-pair donors. This theory provides the most general definition of acids and bases and can be applied to a wide range of chemical reactions.
Properties of Acids and Bases
Acids and bases have distinct properties that can be used to differentiate them. Some of the common properties are summarized in the following table:
| Property | Acids | Bases |
|---|---|---|
| Taste | Sour | Bitter |
| pH | Less than 7 | Greater than 7 |
| Reaction with metals | Produce hydrogen gas | No reaction |
| Reaction with carbonates | Produce carbon dioxide gas | No reaction |
| Conductivity of electricity | Good conductors | Good conductors |
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are basic.
Neutralization reactions occur when an acid and a base react to form a salt and water. These reactions are typically exothermic, releasing heat.
Acid-Base Reactions: Pogil Acids And Bases Answer Key
Acid-base reactions are chemical reactions that involve the transfer of protons or electrons between acids and bases. These reactions can be used to determine the concentration of acids or bases in a solution through a process called titration.
Titration involves the gradual addition of a known concentration of base to an acid solution until the reaction is complete. The endpoint of the titration is reached when the pH of the solution reaches a specific value, which is typically indicated by a color change of an indicator.
Indicators are weak acids or bases that change color depending on the pH of the solution. They are used in titrations to signal the endpoint of the reaction.
Applications of Acids and Bases
Acids and bases have a wide range of industrial and everyday applications. Some of the common applications include:
- Industrial applications: Acids and bases are used in the production of fertilizers, plastics, dyes, and other chemicals.
- Everyday applications: Acids and bases are used in household cleaning products, batteries, and food preservatives.
- Biological applications: Acids and bases play a crucial role in maintaining the pH balance of living organisms and are involved in various biochemical reactions.
Popular Questions
What is the Arrhenius theory of acids and bases?
The Arrhenius theory defines acids as substances that produce hydrogen ions (H+) in water, while bases produce hydroxide ions (OH-) in water.
What is the difference between a strong acid and a weak acid?
Strong acids completely dissociate in water, releasing all their hydrogen ions, while weak acids only partially dissociate, releasing only a fraction of their hydrogen ions.
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 (most acidic) to 14 (most basic), with 7 being neutral.